The Role of Surfactants in Cleaning

When we clean our homes, workplaces, or even ourselves, the role of surfactants often goes unnoticed. However, these powerful chemical agents are at the core of nearly every cleaning product you use. Whether you're dealing with grease on your kitchen stove, oil stains in your laundry, or simply washing your hands, surfactants are what make these tasks easier and more efficient.
In this in-depth article, we will explore the fascinating chemistry of surfactants, explaining exactly how they work, the different types available, and why they are essential for effective cleaning. If you're curious about the science behind cleaning or looking for information on how to choose the right cleaning products for your needs, you're in the right place. We’ll also address common concerns such as the environmental impact of surfactants and provide tips on selecting eco-friendly alternatives.
What Are Surfactants?
At their most basic level, surfactants, or surface-active agents, are chemical compounds that reduce the surface tension between two substances, such as oil and water. This ability to lower surface tension is what makes surfactants so effective in cleaning. Without them, water alone would struggle to remove greasy or oily dirt from surfaces since oil and water don’t naturally mix.
To understand surfactants better, it's crucial to look at their structure. Each surfactant molecule is made up of two distinct parts:
The Hydrophilic Head: This is the water-attracting (water-loving) portion of the molecule, which readily bonds with water molecules.
The Hydrophobic Tail: This is the water-repelling (water-hating) portion, which binds to oils, fats, and grease instead of water.
This dual nature of surfactants allows them to act as a bridge between water and oils. When you apply a cleaning product containing surfactants, the hydrophobic tails bind to the dirt, while the hydrophilic heads remain in the water. This forms structures called micelles that trap the dirt, making it easier to wash away.
By breaking down the molecular barriers between water and oil, surfactants enable the effective removal of stubborn stains, grease, and grime from surfaces, fabrics, and skin. It's this unique ability that makes them indispensable in everything from dish soaps to shampoos and household cleaners.

Why Are Surfactants Essential for Cleaning?
Understanding how surfactants work allows us to appreciate why they are found in virtually every cleaning product. Surfactants are essential because they solve one of the biggest challenges in cleaning: the removal of greasy, oily dirt that doesn't dissolve in water. Water on its own is an excellent solvent for many substances, but when it comes to grease or oil-based grime, it falls short. This is where surfactants come in.
Their ability to reduce surface tension and create emulsions means that they allow water to mix with oils and fats, lifting them away from the surface you're cleaning. This process makes it possible for even simple water-based cleaners to tackle heavy-duty tasks such as cleaning greasy stovetops or removing oil stains from clothing.
Moreover, surfactants not only improve the efficiency of cleaning but also make the cleaning process faster. By lowering the energy required to remove dirt, they save you time and effort. Instead of scrubbing relentlessly, surfactant-based cleaners do the heavy lifting for you, allowing for faster and more thorough cleaning results.
Types of Surfactants and Their Uses
Surfactants come in various types, each with its specific characteristics, strengths, and ideal applications. Knowing the difference between these types can help you choose the right product for your particular cleaning task.
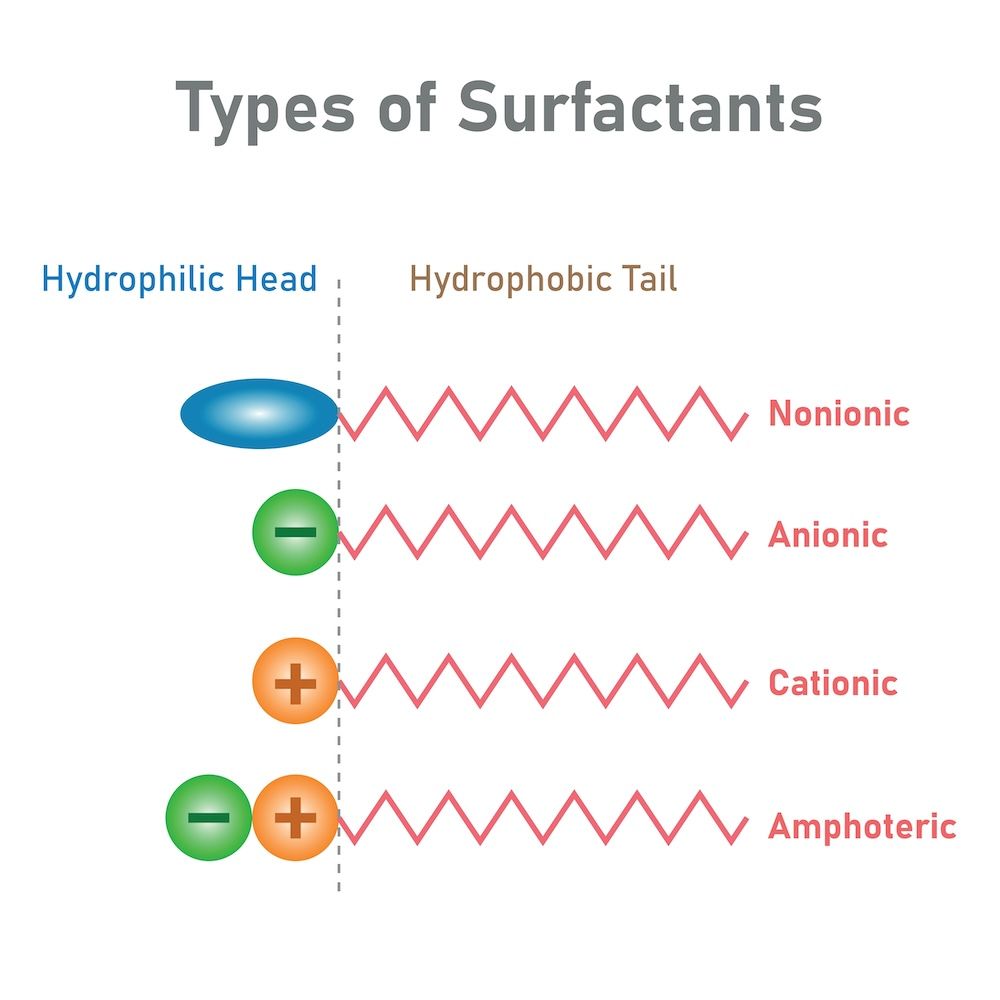
1. Anionic Surfactants
These are negatively charged surfactants, and they are particularly effective at lifting oily dirt and grease. This makes them common in heavy-duty cleaning products, such as laundry detergents and dishwashing liquids. However, their high effectiveness comes with the downside of potentially being too harsh for delicate surfaces or skin. Anionic surfactants are highly effective at breaking down oils, which is why they are the go-to choice for many household cleaning products.
Common examples: Sodium lauryl sulfate (SLS), found in soaps and shampoos, is a widely used anionic surfactant.
2. Nonionic Surfactants
These surfactants carry no electrical charge, making them milder than their anionic counterparts. They are perfect for cleaning surfaces that need gentle care, such as glass, ceramic, and certain types of flooring. Despite their mildness, nonionic surfactants are still highly effective at removing dirt and grime, particularly in situations where a softer touch is required. You will often find them in household cleaning products designed for more delicate tasks, like window cleaners and furniture polish.
Common examples: Alcohol ethoxylates, often found in household cleaners and dish soaps, are effective nonionic surfactants.
3. Cationic Surfactants
These positively charged surfactants are commonly used in products that need both cleaning and disinfecting properties. Cationic surfactants have antimicrobial properties, which make them highly effective in killing bacteria and other microorganisms. This is why they are often found in disinfectants, fabric softeners, and even personal care products like conditioners. If you're cleaning in an environment that requires both cleanliness and sanitization—such as a kitchen, bathroom, or healthcare setting—cationic surfactants are often the best choice.
Common examples: Quaternary ammonium compounds (quats), which are found in disinfectants and fabric softeners, are a type of cationic surfactant.
4. Amphoteric Surfactants
Amphoteric surfactants can act as either anionic or cationic, depending on the pH of the solution they are in. This versatility makes them incredibly useful in a wide range of cleaning applications. You’ll find amphoteric surfactants in products that need to adapt to varying cleaning environments, such as personal care products like shampoos and body washes, as well as in industrial cleaning solutions. Their ability to work in both acidic and alkaline conditions makes them versatile and highly adaptable.
Common examples: Cocamidopropyl betaine, a gentle surfactant often found in body washes and shampoos.
How Surfactants Work in Cleaning: A Closer Look
When you apply a cleaning solution that contains surfactants to a surface, several processes occur at the molecular level. First, the surfactant molecules position themselves at the interface between the water and the grease or dirt. The hydrophobic tails bind to the oil or grease, while the hydrophilic heads remain in the water. This forms micelles—tiny spheres where the oily dirt is trapped inside, surrounded by water. Once these micelles have formed, the dirt and grease can be easily rinsed away, leaving the surface clean.
The ability of surfactants to form micelles is what makes them so effective at removing even the most stubborn grime. Whether you are cleaning a greasy pan, an oily stain from your clothes, or just trying to get your hands clean after working on your car, surfactants make the job much easier by ensuring that water and oil-based dirt can mix and be washed away.

Environmental Considerations: Choosing Eco-Friendly Surfactants
In recent years, there has been growing concern about the environmental impact of surfactants. Traditional surfactants are often derived from petroleum-based sources, which are not biodegradable and can harm aquatic ecosystems when they enter waterways. The environmental impact of these surfactants can be long-lasting, as they persist in the environment and can disrupt ecosystems.
However, many manufacturers are now turning to more eco-friendly alternatives. Plant-based surfactants, derived from renewable resources such as coconut oil or palm oil, offer a more sustainable option. These surfactants are biodegradable and break down naturally in the environment, minimizing their ecological footprint. When shopping for cleaning products, look for those labeled as biodegradable or plant-based to ensure that you're choosing products that are both effective and environmentally responsible.
Tips for Selecting the Right Surfactant-Based Cleaners
When it comes to choosing the right cleaning products for your home or workplace, understanding the role of surfactants can help you make smarter, more informed decisions. Here are a few tips to keep in mind:
For tough, greasy stains, choose products with anionic surfactants. These are ideal for tasks such as cleaning kitchen countertops, stovetops, and removing oil stains from clothing.
For delicate surfaces, such as glass, ceramics, or hardwood floors, opt for cleaners with nonionic surfactants. These will clean effectively without damaging the surface or leaving streaks.
For disinfecting and sanitizing, look for products containing cationic surfactants. These are perfect for cleaning bathrooms, kitchens, and other areas where hygiene is a priority.
For eco-friendly cleaning, choose products with biodegradable surfactants. These plant-based alternatives are just as effective as traditional surfactants but come with the added benefit of being better for the environment.
Surfactants as the Unsung Heroes of Cleaning
Surfactants are at the heart of modern cleaning products, playing a crucial role in breaking down grease, grime, and stains so that they can be easily removed with water. Whether you're cleaning your home, washing your clothes, or scrubbing a commercial kitchen, understanding how surfactants work and how to choose the right one can make your cleaning routine more efficient and environmentally friendly.
By leveraging the science of surfactants, you can achieve better cleaning results with less effort, all while making choices that are kind to the environment. Whether you're a professional cleaner or simply someone who wants to keep your home in top shape, surfactants are the key to smarter, more effective cleaning.
Check more articles on our blog

Effective and Eco-Friendly Home Cleaning with Baking Soda

Cleaning Products Recommended by Service Providers Cooperating with SPIC AND SPAN. Home & Office Cleaning

Explore Brilliant Natural Methods To Keep Your Home Clean
